Food Supplement vs Dietary Supplement: Key Differences & Compliance

According to the CDC, over 57% of U.S. adults consume dietary supplements, with usage significantly increasing among older adults. The market is booming, yet many consumers remain unclear about the distinction between food supplements and dietary supplements. While these terms are often used interchangeably, they hold different meanings under various regulatory frameworks. Understanding these differences is crucial, especially for businesses involved in the nutraceutical industry.
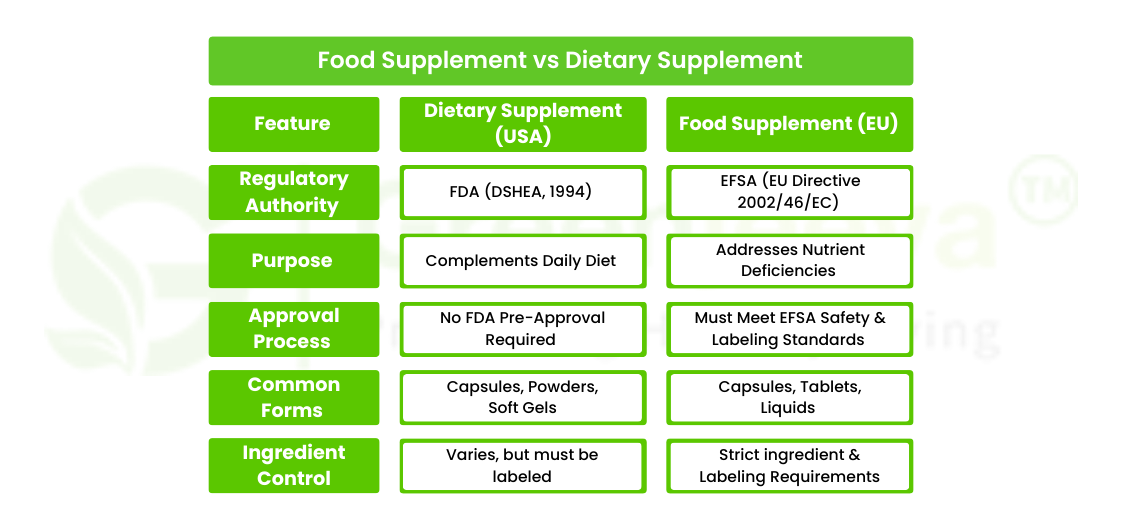
What Is a Dietary Supplement?
According to the FDA, a dietary supplement is a product that is intended to supplement the diet and contains one or more dietary ingredients such as:
• Vitamins and minerals
• Herbs and botanicals
• Amino acids
• Probiotics and enzymes
• Other dietary substances
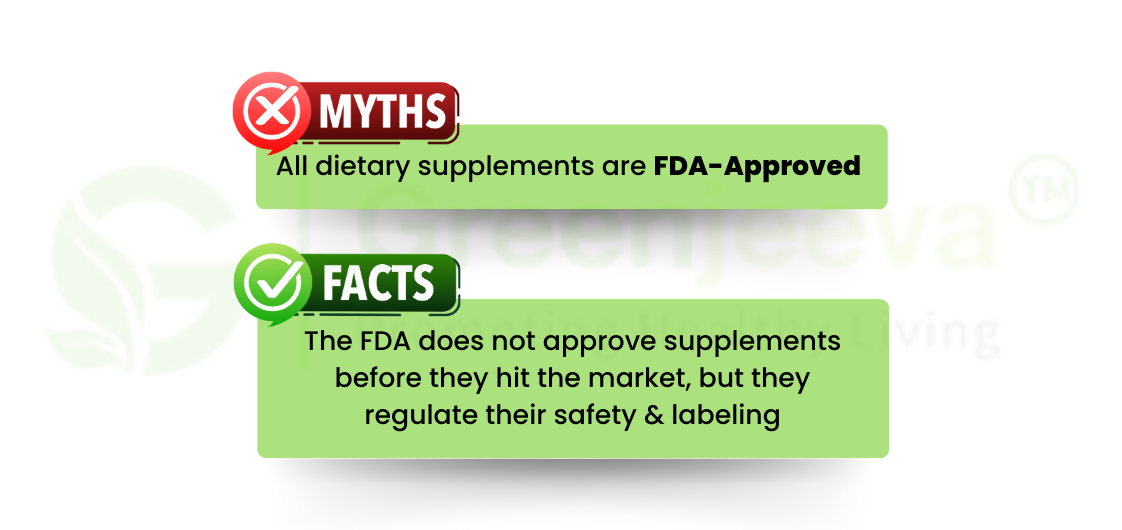
In the United States, dietary supplements are regulated under the Dietary Supplement Health and Education Act (DSHEA) of 1994. Dietary supplements come in various forms, including tablets, capsules, powders, and liquids. Unlike prescription drugs, they do not require FDA approval before entering the market, but manufacturers must ensure their safety and accurate labeling.
What Is a Food Supplement?
The term food supplement is more commonly used in European markets. Under EU Directive 2002/46/EC, food supplements are defined as concentrated sources of nutrients marketed in dose forms such as capsules, tablets, or liquids. The primary purpose of food supplements is to correct nutritional deficiencies or maintain an adequate nutrient intake. These supplements must comply with strict ingredient and labeling regulations set by the European Food Safety Authority (EFSA).
Supplemental Compliance: Ensuring Safety and Quality
Given the increasing demand for supplements, supplemental compliance is a crucial aspect that both manufacturers and consumers must prioritize.
Compliance Standards in the U.S.
1. Good Manufacturing Practices (cGMPs): Manufacturers must follow FDA-enforced cGMPs to ensure product quality, purity, and labeling accuracy.
2. Labeling Requirements: Supplements must clearly state ingredients, serving size, and a disclaimer that the FDA has not evaluated the product.
3. Adverse Event Reporting: Manufacturers must report serious health-related incidents linked to supplement use to the FDA.
Also Know More:Why Cupuacu Powder is the New Darling of Nutraceuticals
Compliance Standards in the EU
1. Ingredient Approval: Only approved vitamins, minerals, and substances can be included in food supplements.
2. Health Claims Regulation: Any claims made must be substantiated with scientific evidence and approved by the EFSA.
3. Traceability Measures: Products must maintain full traceability of ingredients, from sourcing to consumer distribution.
Also Know More: Best Tips to Shop for Safe Dietary Supplements
Why Compliance Matters for Businesses and Consumers
Non-compliance can lead to regulatory action, product recalls, and reputational damage. Consumers should verify certifications and look for third-party testing to ensure product quality.
How Green Jeeva Supports Compliance in the Nutraceutical Industry
Green Jeeva simplifies sourcing for dietary supplements by ensuring strict compliance with global standards. With transparent documentation, real-time stock updates, and a diverse range of 1,400+ premium ingredients, businesses can source with confidence.
Conclusion
Understanding the distinction between food supplements and dietary supplements is essential for manufacturers, retailers, and consumers. While both aim to provide essential nutrients, their regulatory requirements and market positioning differ. Ensuring supplemental compliance not only safeguards businesses from legal risks but also protects consumers from potential health hazards.
FAQs:
Are dietary supplements and food supplements the same?
No. While both provide essential nutrients, dietary supplements are common in the U.S., while food supplements follow EU regulations.
Do dietary supplements need FDA approval?
No. Dietary supplements do not require FDA pre-approval but must meet safety and labeling standards under the Dietary Supplement Health and Education Act (DSHEA).
Can I take food supplements and dietary supplements together?
Yes, but you should avoid exceeding recommended daily intake levels. Always consult a healthcare professional before combining multiple supplements.
Are there risks associated with dietary or food supplements?
Yes. Risks include overconsumption, harmful interactions, or contaminants. Always choose certified and third party-tested products.
How do I verify supplement quality before purchasing?
Look for Certifications (USDA Organic, Non-GMO, GMP-certified), transparent ingredient sourcing, and third-party lab test results before purchasing.
Also read - Everything You Need to Know About Food Additives and Preservatives
**The Food and Drug Administration has not evaluated these statements. This product is not intended to diagnose, treat, cure, or prevent any disease.**



.jpg)



