Formulating Pre and Probiotic Supplements: Key Strategies for Quality Ingredient Sourcing

In the competitive world of supplement manufacturing, pre and probiotic supplements have seen a surge in demand due to their proven health benefits, particularly in digestive and immune health. However, the growing popularity has also raised the bar for quality standards, making ingredient sourcing and stringent quality control essential for manufacturers looking to stand out. This guide outlines the best practices for sourcing high-quality prebiotics and probiotics, focusing on ingredient purity and ensuring compliance with industry standards, with an emphasis on the increasingly popular strains like Lactobacillus.
The Importance of High-Quality Pre and Probiotic Ingredients
The foundation of any successful pre and probiotic supplement is the quality of its ingredients. Prebiotics, which act as food for beneficial bacteria, and probiotics, which consist of live bacterial strains like Lactobacillus, must be carefully selected to ensure they offer the desired health benefits. Inferior or contaminated raw materials can result in ineffective products, reputational damage, and potential regulatory issues.
A report from Transparency Market Research projects the global probiotic market to grow at a compound annual growth rate (CAGR) of 8.3% from 2020 to 2030. With demand accelerating, sourcing ingredients that meet quality benchmarks is not just important for product efficacy but also for regulatory compliance and consumer trust.
The Role of Lactobacillus in Probiotic Supplements
One of the most widely researched probiotic strains, Lactobacillus plays a significant role in gut health, supporting digestion and the immune system. For manufacturers, the inclusion of Lactobacillus strains is not only beneficial due to their effectiveness but also due to consumer recognition, which can boost sales.
According to a report by Grand View Research, Lactobacillus supplements account for a significant portion of the probiotic market, driven by rising consumer awareness and the growing demand for gut health products. Manufacturers should ensure that they source high-quality Lactobacillus strains, specifically strains with robust clinical support, to stay competitive.
Best Practices for Sourcing Prebiotic Ingredients
When sourcing prebiotic ingredients such as inulin, fructooligosaccharides (FOS), or galactooligosaccharides (GOS), manufacturers should focus on:
- Traceability: Sourcing from certified suppliers with clear documentation of origin ensures transparency and reduces the risk of contamination.
- Purity: Prebiotics should be free from harmful additives or chemicals that could impact the product's quality.
- Solubility and Stability: Prebiotics should be compatible with probiotic strains and remain stable during production, packaging, and storage.
In addition, prebiotic ingredients must meet specific industry guidelines, such as those from the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA), to ensure their safety and effectiveness in supplement formulations.
Sourcing High-Quality Probiotics: Key Considerations
For probiotics, such as Lactobacillus acidophilus and Bifidobacterium bifidum, sourcing high-quality strains involves a more complex process due to their live nature. Here are some crucial aspects manufacturers should consider:
Strain Identification and Selection: Each probiotic strain offers distinct benefits. The efficacy of Lactobacillus and other strains depends on proper strain identification and compatibility with prebiotic ingredients.
CFU Count (Colony-Forming Units): CFU count is essential for ensuring probiotic efficacy. Manufacturers must ensure that the live bacteria count is maintained through production and until the end of the product's shelf life. Partnering with suppliers who guarantee the delivery of a specified CFU count is critical for product quality.
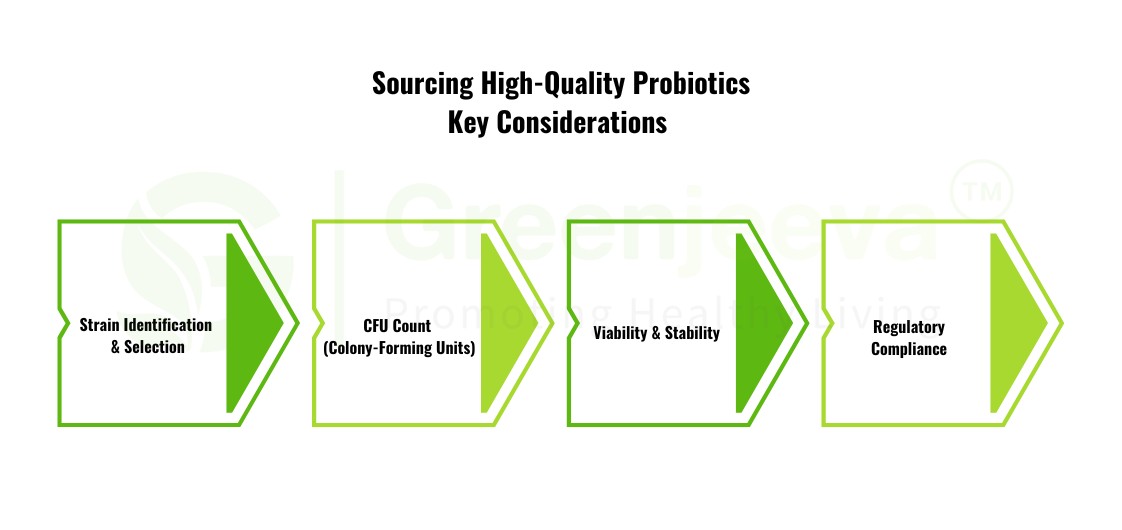
Viability and Stability: Probiotic strains must survive manufacturing processes and remain stable during the product’s shelf life. Look for suppliers that offer microencapsulated or freeze-dried probiotics to enhance their stability.
Regulatory Compliance: Ensure that probiotic strains are GRAS (Generally Recognized As Safe) certified, as non-compliance can lead to costly recalls and potential legal consequences.
Quality Control in Pre and Probiotic Supplement Manufacturing
Quality control is essential throughout the production process to ensure that pre and probiotic supplements meet efficacy, safety, and regulatory requirements. Key quality control measures include:
- Raw Material Testing: Manufacturers should test all raw ingredients for contaminants, including heavy metals, pesticides, and microbial impurities.
- In-Process Monitoring: During the production phase, manufacturers must monitor the temperature, moisture, and microbial counts to maintain probiotic viability and prevent degradation of prebiotics.
- Shelf-Life Testing:Probiotic supplements must be tested to confirm that they maintain their stated CFU counts up to the product’s expiration date.
- Third-Party Testing and Certifications: Partnering with third-party laboratories for independent testing ensures transparency and builds consumer trust. Certifications from organizations like NSF International or the United States Pharmacopeia (USP) can further validate product quality.
Industry Trends and Market Opportunities
As the global market for pre and probiotic supplements continues to expand, driven by rising consumer awareness of gut health, manufacturers have an opportunity to tap into emerging trends:
- Personalized Nutrition: Consumers are seeking supplements tailored to their individual health needs, prompting the demand for specific probiotic strains like Lactobacillus rhamnosus and Bifidobacterium longum.
- Plant-Based Probiotics: With the rise of plant-based diets, there is increasing interest in plant-derived probiotic strains, offering manufacturers a new niche market.
- Regulatory Compliance: Strict regulatory standards in regions like the EU and the US mean that compliance with quality standards is crucial for market access. Staying informed about regulatory changes and ensuring ingredient compliance is key for maintaining product credibility.
Also Know More: A Global Perspective on Cross-Border Ingredient Sourcing
Conclusion
Manufacturers formulating pre and probiotic supplements must prioritize ingredient sourcing and quality control to create effective, safe, and compliant products. By focusing on the purity of prebiotics, the viability of probiotics like Lactobacillus, and stringent quality measures, supplement manufacturers can meet rising consumer expectations and industry standards. As demand for these supplements grows, maintaining high-quality sourcing and production standards will be essential for long-term success in the market.
**The Food and Drug Administration has not evaluated these statements. This product is not intended to diagnose, treat, cure, or prevent any disease.**






